
#Hydrophobic amino acids and neutral polar amino acids code#
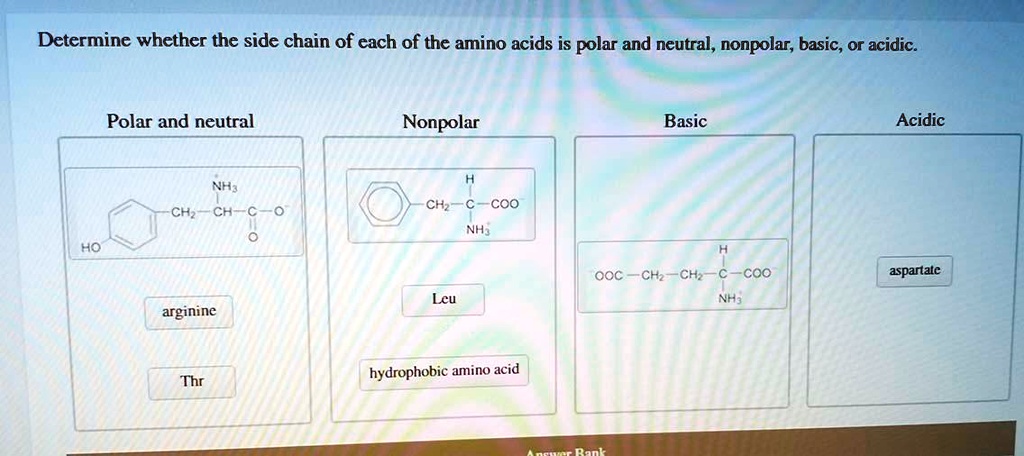
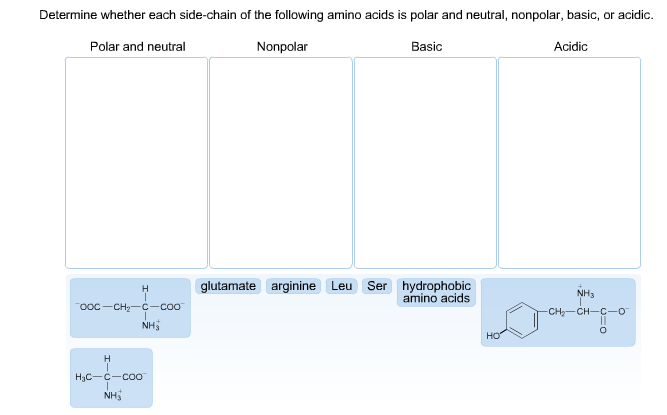
To learn more about an amino acid, to find out which aminoĪcid a one letter code abbreviates, and to veiw its structure click The structures of the side chains and basic principles of organicĬhemistry. You should not need to memorize the list,īut should be able to predict the properties from your knowledge of The R-groups can be classified in many different ways, several of Twenty amino acids which are present in newly synthesized proteins. Remind you of the properties of the side chains/R-groups of the So, as you can see, even though we’ve come a long way in our understanding of amino acids and the important roles they play in the human body, we still have more to learn before we can say we've truly mastered all the intricacies of these essential building blocks of life.This list is provided as a study guide to help In fact, where amino acids are concerned, researchers continue to disagree on several points, including whether certain forms should be considered polar or hydrophilic. You see, cysteine is classified as only slightly polar and thus doesn’t fit well into either the polar or non-polar category. While it may have looked like we were bad at math, we promise we really knew what we were doing when we left cysteine (Cys) out of our lists. You may have noticed that we mentioned 20 common amino acids earlier but listed only 19. Like the rest of the common amino acids, the neutral, acidic, and basic polar groups all perform important functions in the body. In the case of polar amino acids, this refers to the various combinations of amide and carboxylic acid groups that interact to create the neutral, acidic, and basic forms. The degree of polarity is also determined by the functional groups-the groups of atoms that dictate the chemical behavior of a compound-contained in the side chains.
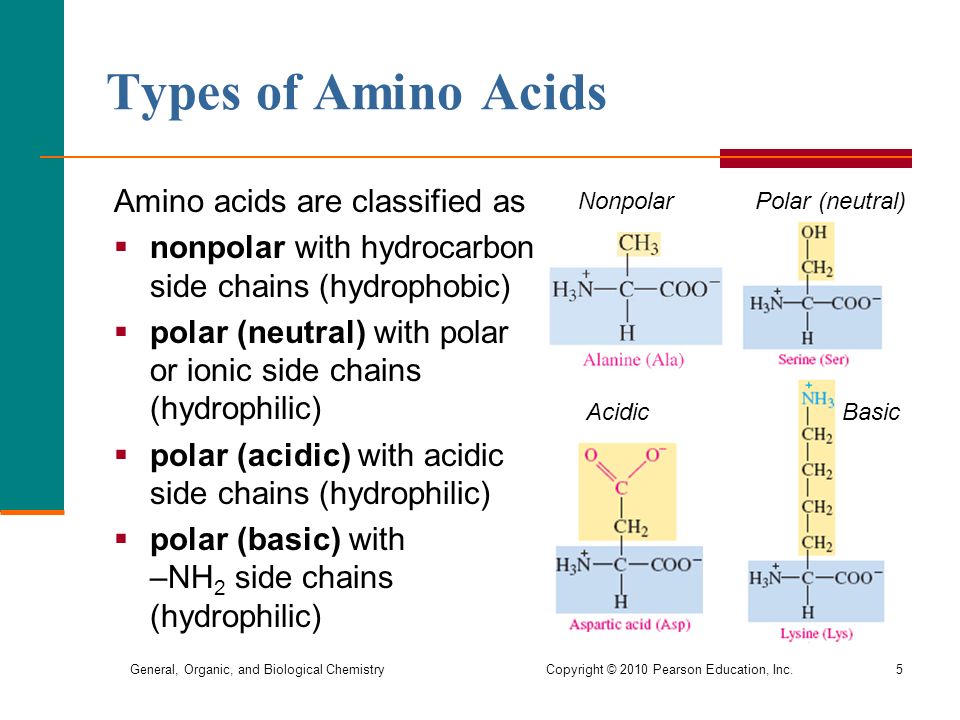
Arginine, histidine, and lysine are basic amino acids and have a positive charge. If the side chain contains an extra nitrogen group, the amino acid becomes basic. The aspartate and glutamate types are the anions, or negatively charged ions, of these substances. These forms are also known as aspartic acid and glutamic acid, respectively.
Aspartate and glutamate are acidic amino acids. If the side chain contains an extra element of carbolic acid, the amino acid becomes acidic. Of the polar amino acids, asparagine, glutamine, serine, threonine, and tyrosine are neutral. The majority of amino acids, both polar and non-polar, are in fact neutral. This means that their side chains contain exactly one amino group and one carboxyl group (hence the name "amino acid"). Neutral Polar GroupsĪs the name suggests, neutral polar amino acids are neither basic nor acidic. And each of these categories functions in a different way. The polar amino acids can be further broken down into neutral, basic, and acidic groups. What Are the Properties of Polar Amino Acids? Including their three-letter codes, these amino acids are: Arginine (Arg) These 20 amino acids are known as the common amino acids. However, only 20 are used to synthesize proteins. There are many different amino acids, with over 300 known forms listed in the Practical Handbook of Biochemistry and Molecular Biology. In this case, instead of dissolving, the presence of the oil results in the amino acids being attracted to each other. Interestingly, although the hydrophilic nature of polar amino acids means that they readily dissolve in water, they actually have the opposite reaction when placed in oil. Because of this water-loving characteristic, these amino acids are generally located on the surface of proteins, in contact with the aqueous cell environment. In contrast, polar amino acids have hydrophilic side chains, which means they’re actually attracted to water and participate in hydrogen bonding with the highly polar water molecules. These amino acids are thus located in the protein core, safely tucked away from any contact with water. The non-polar groups are hydrophobic amino acids, which means they have side chains that are repelled by water. And the shape of the protein-yes, proteins really do have shape-is determined mainly by the sequence of amino acids in that chain. When amino acids join together to form proteins, only their side chain groups are exposed and able to interact with each other and their surroundings. But did you know there are actually two types? Known as non-polar and polar amino acids, each group is classified according to its side chains-the shorter chains of atoms attached to the main chain, or backbone, of a molecule. When most people think of amino acids, they probably have a vague memory of their high school science teacher discussing the building blocks of protein.


 0 kommentar(er)
0 kommentar(er)
